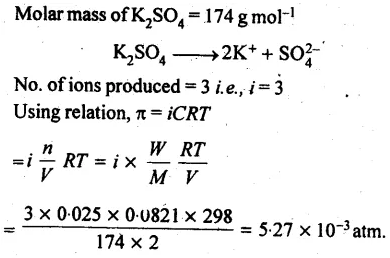Chapter 2 Solutions
NCERT IN-TEXT QUESTIONS
Question 1.
Answer:
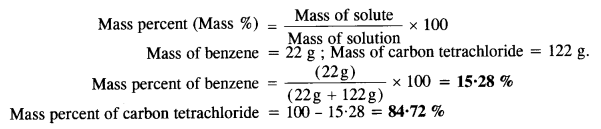
Question 2.
Calculate the mole fraction of benzene in a solution containing 30% by mass of it in carbon tetrachloride.
Answer:
Let us start with 100 g of the solution in which
Mass of benzene = 30 g
Mass of carbon tetrachloride = 70 g
Molar mass cf benzene (C6H6) = 6 x 12 + 6 x 1 = 78g mol-1.

Question 3.
![]()
Answer:

= 0.03M
Question 4.
![]()
Answer:
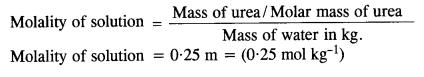

Question 5.
Calculate (a) molality (b) molarity and (c) mole fraction of KI if the density of 20% (mass/mass) aqueous KI is 1.202 g mL-1 .
Answer:

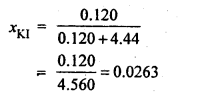
Question 6.
H2S a toxic gas with rotten egg smell, is used for the qualitative analysis. If the solubility of H2S in water at S.T.P is 0·195 m ; calculate Henry’s law constant.
Answer:
Step I. Calculation of mole fraction of H2S
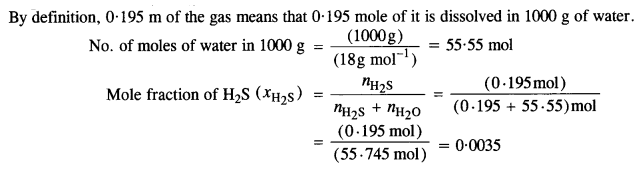
Step II. Calculation of Henry’s Law constant
According to Henry’s Law,
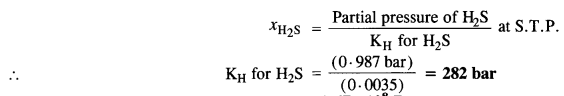
Question 7.
Henry’s Law constant for CO2 in water is 1·67 x 108 Pa at 298 K. Calculate the quantity of CO2 in 500 mL of soda water when packed under 2·5 atm pressure of CO2 at 298 K. (D.S.B. 2008 Supp.)
Answer:
Step I. Calculation of number of moles of CO2.
According to Henry’s Law,

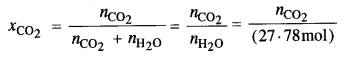
(nCO2 has been neglected as the gas is very little soluble in water)
∴ nCO2 = xCO2 x (27·78 mol) = (1·52 x 10-3) x (27·78 mol) = 0·0422 mol
Step II. Mass of CO2 dissolved in water = (0·0422 mol) x (44 g mol-1) = 1·857 g.
Question 8.
The vapour pressure of pure liquids A and B are 450 and 700 mm Hg respectively, at 350 K. Find out the composition of the liquid mixture if total vapour pressure is 600 mm Hg. Also find the composition of the vapour phase.
Answer:
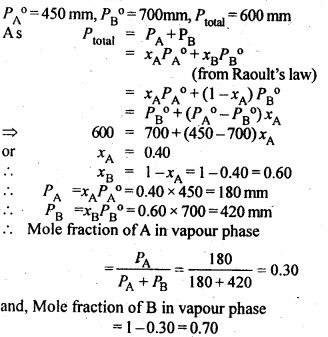
Question 9.
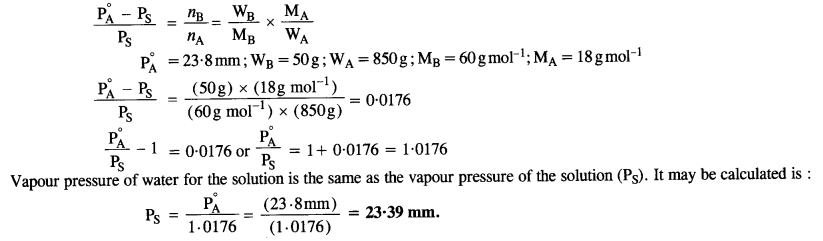
The vapour pressure of pure water at 298 K is 23·8 mm Hg. 50 g of Urea (NH2CONH2) is added to 850 g of water. Calculate the vapour pressure of water for this solution and also it’s relative lowering in vapour pressure.
Answer:
Step I. Calculation of vapour pressure of water for the solution
According to Raoult’s Law,
Step II. Calculation of relative lowering in vapour pressure
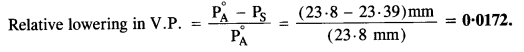
Question 10.
The boiling point of water at 750 mm Hg is 99·63°C. How much sucrose is to be added to 500 g of water so that it may boil at 100°C? (K6 for water = 0·52 K kg mol-1).
Answer:

Question 11.
![]()
Answer:

Question 12.
Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 1·0 g of a polymer of molar mass 185,000 in 450 mL of solution at 37°C.
Answer:

NCERT EXERCISE
Question 1.
Define the term solution. What kinds of solutions are possible? Write briefly about each type of solution with an example.
Answer:
A true solution is a homogenous mixture of two or more substances. The constituent particle which is in larger amount’’ is called a solvent and that in smaller quantity is called a solute.
TYPES OF SOLUTION

Question 2.
Give an example of a solid solution in which the solute is a gas.
Answer:
Solid in solid type. E.g: Copper in gold. This type of solutions are called alloys.
Question 3.
Define the following terms :
(i) Mole fraction
(ii) Molality
(iii) Molarity
(iv) Mass percentage.
Answer:
(i) Mole fraction :
The ratio of the number of moles of one component to the total number of moles of all the components present in the solution.
For a binary solution made up of components A and B,
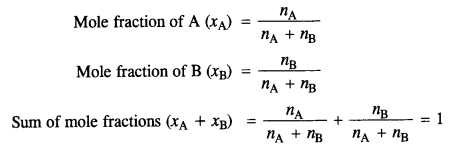
(ii) Molality:
The number of gram moles of the solute dissolved in 1000 g (or kg) of the solvent.

(iii) Molarity:
The number of gram formula mass of the solute dissolved per litre of the solution.

(iv) Mass percentage:
The number of parts by mass of one component (solute or solvent) per 100 parts by mass of the solution. If A and B are the two components of a binary solution,

Question 4.
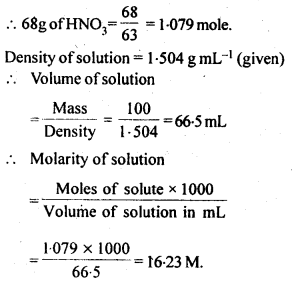
Concentrated nitric acid used in laboratory work is 68% nitric acid by mass in an aqueous solution. What should be the molarity of such a sample of the acid if the density of the solution is 1.504 g mL-1?
Answer:
68% nitric acid by mass means that 68g mass of nitric acid is dissolved in 100g mass of solution. Molar mass of HNO3= 63g mol-1
Question 5.
A solution of glucose in water is labelled as 10 percent W/W. What would be the molality and mole fraction of each component in the solution? If the density of the solution is 1·2 g mL-1, then what should be the molarity of the solution? (C.B.S.E. 2013, Manipur Board 2015)
Answer:

Question 6.
How many mL of a 0·1 M HCl are required to react completely with a 1 g mixture of Na2CO3 and NaHCO3 containing equimolar amounts of two?
Answer:
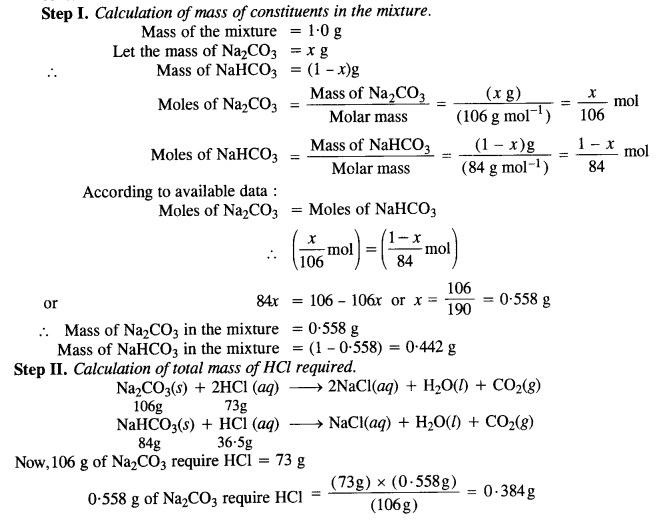

Question 7.
A solution is obtained by mixing 300 g of 25% solution and 400 g of 40% solution by mass. Calculate the mass percentage of the resulting solution.
Answer:

Question 8.

Answer:
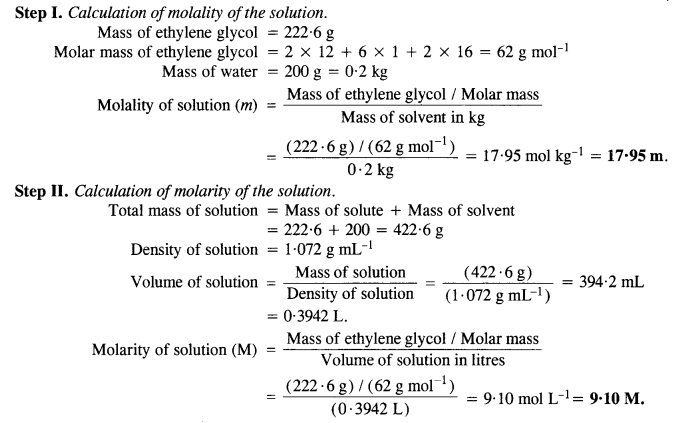
Question 9.
A sample of drinking water was found to be severely contaminated with chloroform(CHCl3) supposed to be a carcinogen. The level of contamination was 15 ppm (by mass).
(i) Express this in percent by mass.
(ii) Determine the molality of chloroform in a water sample.
Answer:

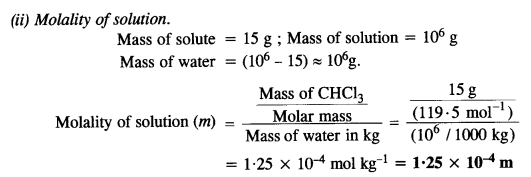
Question 10.
What role does the molecular interaction play in a solution of alcohol and water?
Answer:
Alcohols dissolve in water due to the formation of intermolecular H-bonding with water.
Question 11.
Why do gases nearly always tend to be less soluble in liquids as the temperature is raised?
Answer:
The dissolution of a gas in a liquid is exothermic in nature because the gas contracts in volume.

An increase in temperature will favour the reverse process since it is of endothermic nature. Therefore, the solubility of the gas in the solution decreases with the rise in temperature.
Question 12.
State Henry’s law and mention some important applications.
Answer:
Henry’s law: According to this law, ‘The mass of a gas dissolved per unit volume of a solvent at a constant temperature, is proportional to the pressure of the gas with which the solvent is in equilibrium’.
Let in unit volume of solvent, the mass of the gas dissolved is m and equilibrium pressure is P, then m α P or m = KP, where K is a constant. We can understand Henry’s law by taking the example of soda water bottle. Soda water contains carbon dioxide dissolved in water under pressure.
Applications of Henry’s law:
1. In the production of carbonated beverages: To increase the solubility of CO2 in soft drinks, soda water, bear etc. the bottles are sealed at high pressure.
2. In exchange of gases in the blood: The partial pressure of O2 is high in inhaled air, in lungs it combines with hemoglobin to form oxyhemoglobin. In tissues, the partial pressure of oxygen is comparatively low therefore oxyhemoglobin releases oxygen in order to carry out cellular activities.
3. In deep-sea diving: Deep-sea divers depend upon compressed air for breathing at high pressure underwater. The compressed air contains N2 in addition to O2, which are not very soluble in blood at normal pressure. However, at great depths when the diver breathes in compressed air from the supply tank, more N2 dissolved in the blood and in other body fluids because the pressure at that depth is far greater than the surface atmospheric pressure. When the divers come towards the surface at atmospheric pressure, this dissolves nitrogen bubbles out of the blood. These bubbles restrict blood flow, affect the transmission of nerve impulses. This causes a disease called bends or decompression sickness. To avoid bends, as well as toxic effects of high concentration of nitrogen in the blood, the tanks used by scuba divers are filled with air diluted with helium (11.7% He, 56.2% N2, and 32.1% O2).
4. At high altitudes: At high altitudes, the partial pressure of O2 is less than that at the ground level. This results in a low concentration of oxygen in the blood and tissues of the people living at high altitudes or climbers. The low blood oxygen causes climbers to become weak and unable to think clearly known as anoxia.
5. Aquatic life: The dissolution of oxygen (from air) in water helps in the existence of aquatic life in various water bodies like Lake, rivers, and sea.
Question 13.
The partial pressure over a saturated solution containing 6·56 x 10-2 g of ethane is 1 bar. If the solution contains 5·0 x 10-2 g of ethane, what shall be the partial pressure of the gas?
Answer:
According to Henry’s law,
The mass of the gas (m) dissolved in solution ∝ Partial pressure (p) (At constant temperature)
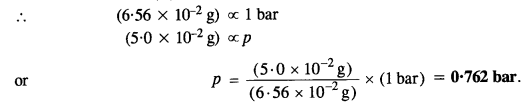
Question 14.
What is meant by positive and negative deviations from Raoult’s law and how is the sign of ∆Hsol related to positive and negative deviations from Raoult’s law?
Answer:
(a) Positive Deviation:
(i) ∆Vmixing is positive: This is quite likely also because in ge presence of weak forces of interaction, interaction, the volume of the solution is bound to increase.
(ii) ∆Hmixing is positive: Energy is needed to form the solution because the components of the solution have to be brought closer to form the solution. Thus, the process of mixing is of endothermic nature.
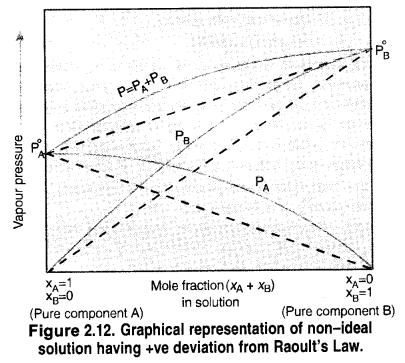
(b) Negative Deviation:
(i) ∆Vmixing is negative: Because of the increased forces of interaction, the molecules of the two components will come closer and as a result, there is a decrease in the volume of the solution.
(ii) ∆Hmixing is negative: Energy is expected to be released because of the increase in the forces of interaction. Therefore, the process of mixing is exothermic in nature or ∆Hmixing is negative.

Question 15.
An aqueous solution of 2% non-volatile solute exerts a pressure of 1.004 bar at the normal boiling point of the solvent. What is the molar mass of the solute?
Answer:

Question 16.
Heptane and octane form ideal solutions. At 373 K, the vapour pressure of the two liquid components is 105·2 k Pa and 46·8 k Pa respectively. If the solution contains 25 g of heptane and 35 g of octane, calculate:
(i) Vapour pressure exerted by heptane
(ii) Vapour pressure exerted by octane
(iii) Vapour pressure exerted by the solution
(iv) Mole fraction of octane in the vapour phase. (C.B.S.E. Sample Paper, 2010)
Answer:

Question 17.
The vapour pressure of water is 12·3 kPa at 300 K. Calculate the vapour pressure of 1 molal solution in it.
Answer:
1 molal solution implies one mole of the solute dissolved in 1000 g (1 kg) of solvent i.e. water.

Question 18.
Calculate the mass of a non-volatile solute (molar mass 40 g mol-1) which should be dissolved in 114 g octane to reduce its vapour pressure to 80%.
Answer:
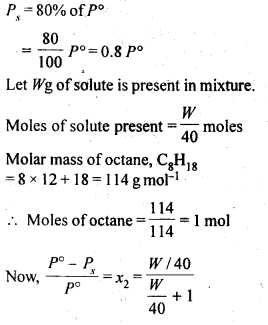
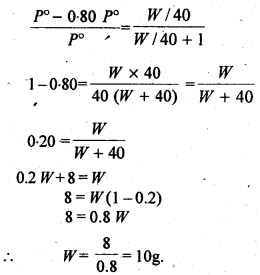
Question 19.
A solution containing 30 g of a non-volatile solute exactly in 90 g of water has a vapour pressure of 2·8 k Pa at 298 K. Further 18 g of water is then added to the solution and the new vapour pressure becomes 2·9 k Pa at 298 K. Calculate
(i) Molecular mass of the solute.
(ii) Vapour pressure of water at 298 K. (C.B.S.E. Outside Delhi 2005)
Answer:



Question 20.
A 5% solution (by mass) of cane sugar in water has a freezing point of 271 K. Calculate the freezing point of a 5% glucose in water if the freezing point of pure water is 273·15 K. (C.B.S.E. Delhi 2008)
Answer:


Freezing point temperature of glucose solution = (273·15 – 4·085) K = 269·07 K.
Question 21.

Answer:
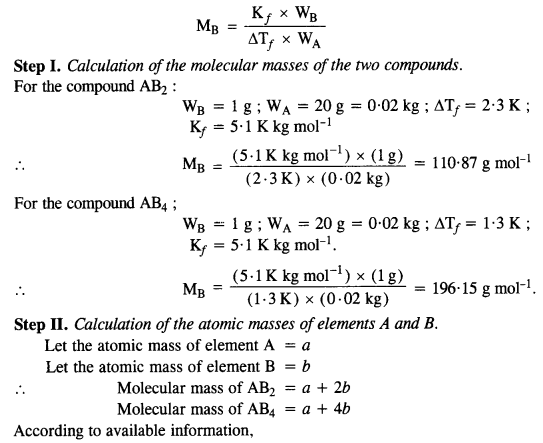
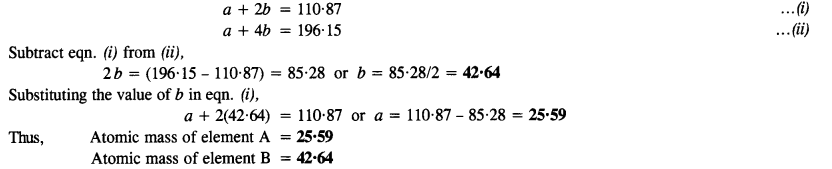
Question 22.
At 300 K, 36g of glucose present in a litre of its solution has an osmotic pressure of 4.08 bar. If the osmotic pressure of the solution is 1.52 bars at the same temperature, what would be its concentration?
Answer:
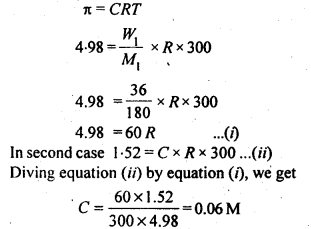
Question 23.
Suggest the most important type of intermolecular attractive interactions in the following pairs :
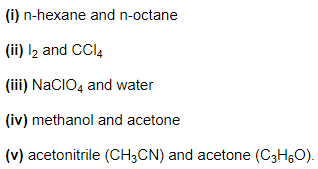
Answer:
- Both are non-polar. Hence, intermolecular interactions in them will be London/ dispersion forces (discussed in class XI)
- Both are non-polar. Hence, intermolecular interactions in them will be London/ dispersion forces (discussed in class XI)
- NaClO4 gives Na+ and ClO4– ions in the solution while water is a polar molecule. Hence, intermolecular interactions in them will be ion-dipole interactions.
- Both are polar molecules. Hence intermolecular interactions in them will be dipole-dipole interactions.
- Both are polar molecules. Hence intermolecular interactions in them will be dipole-dipole interactions.
Question 24.
![]()
Answer:
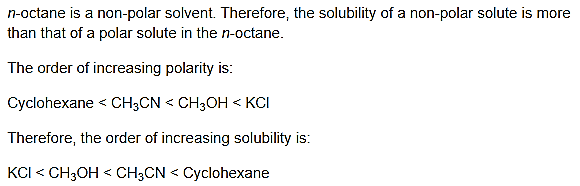
Question 25.
Among the following compounds, identify which are insoluble, partially soluble, and highly soluble in water?
(i) phenol
(ii) toluene
(iii) formic acid
(iv) ethylene glycol
(v) chloroform
(vi) pentanol.
Answer:

Question 26.
![]()
Answer:
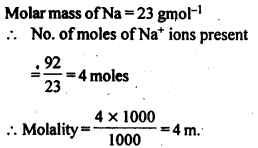
Question 27.
If the solubility product of CuS is 6 x 10-16, calculate the maximum molarity of CuS in aqueous solution.
Answer:
Dissociation of CuS in aqueous solution is :

By definition, Ksp corresponds to the product of the ionic concentration of the salt in saturated solution and it represents the maximum molarity of the salt. Therefore, maximum molarity of the salt = 2· 45 x 10-8 M.
Question 28.
![]()
Answer:

Question 29.

Answer:
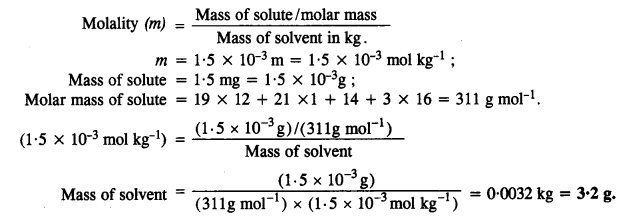
Question 30.
Calculate the amount of benzoic acid (C6H5COOH) required for preparing 250 mL of 0.15 M solution in methanol 0.15 M solution means that 0.15 mole of benzoic acid is dissolved in 1L of solution.
Answer:

Question 31.
The depression in freezing point of water observed for the same amount of acetic acid, trichloroactetic acid and trifluoroacetic acid increases in the order given above. Explain. (C.B.S.E. 2008 Supp.)
Answer:
The depression in freezing point of a solute in water depends upon the number of particles or ions furnished by it in solution or upon its degree of dissociation (α). All the three organic acids ionise in aqueous solution. However, the relative order of acidic strengths is as given below.
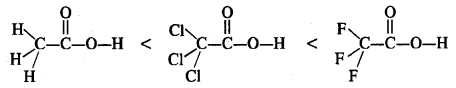
This is linked with the electronegativity of the halogen atoms present. Fluorine (F) is more electronegative than (Cl). Under the circumstances, trifluoroacetic acid gives maximum ions in solution since it is the strongest acid. Consequently, the depression in freezing point (∆Tf) is the maximum in this case and is the least for acetic acid which is the weakest acid.
Question 32.
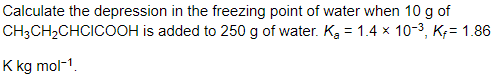
Answer:
Step I. Calculation of degree of dissociation of acid Mass of acid = 10 g

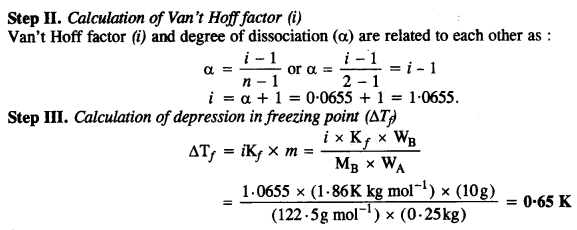
Question 33.
19·5 g of CH2 FCOOH is dissolved in 500 g of water. The depression in the freezing point of water observed is 1·0°C. Calculate Van’t Hoff factor and dissociation constant of the acid: Kf = 1·86 K kg mol-1.
Answer:

Question 36.
100 g of liquid A (molar mass 140 g mol-1) was dissolved in 1000 g of liquid B (molar mass 180 g mol-1). The vapour pressure of pure liquid B was found to be 500 torrs. Calculate the vapour pressure of pure liquid A and its vapour pressure in the solution if the total vapour pressure of the solution is 475 torr.
Answer:

Question 37.


Plot this data also on the same graph paper. Indicate whether it has positive deviation or negative the ideal solution.
Answer:
From the available information:

Since the plot or graph dips downwards, the solution shows a negative deviation from Raoult’s Law.
Question 38.
Benzene and naphthalene form ideal solutions over the entire range of composition. The vapour pressure of pure benzene and naphthalene at 300 K are 50·71 mm Hg and 32·06 mm Hg respectively. Calculate the mole pure fraction of benzene in vapour phase if 80 g of benzene is mixed with 100 g of naphthalene.
Answer:
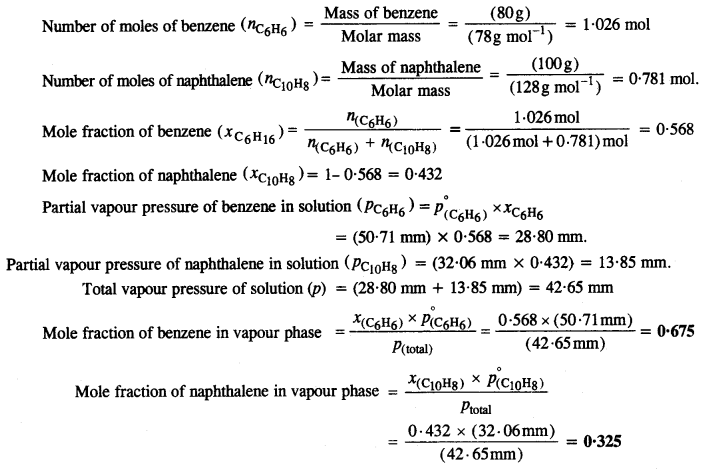
Question 39.
Air is a mixture of a number of gases. The major components are oxygen and nitrogen with the approximate proportion of 20% is to 79% by volume at 298 K. The water is in equilibrium with air at a pressure of 10 atm. At 298 K if Henry’s law constants for oxygen and nitrogen at 298 K are 3·30 x 107 mm and 6·51 x 107 mm respectively, calculate the composition of these gases in water.
Answer:

Question 40.
Determine the amount of CaCl2 (i = 2·47) dissolved in 2·5 litre of water so that its osmotic pressure is 0·75 atm at 27°C.
Answer:
According to Van’t Hoff equation :

Question 41.
Determine the osmotic pressure of a solution prepared by dissolving 25 mg of K2SO4 in 2 litre of water at 25°C, assuming that it is completely dissociated.
Answer: